Emitech’s laboratory in Chassieu, France, accredited by Cofrac, together with the laboratory in Montpellier constitute the group’s medical pole. The centre has testing capabilities for EMC, radio, and electrical equipment safety. This triple competence allows it to support its customers in the procedures for marketing equipment in Europe with CE marking, and throughout the world with its role as CBTL in the context of the CB scheme. These procedures now include on-site incorporation tests, both in France and internationally. Among the various sectors concerned by these regulations, the medical sector is subject to particular requirements both in terms of tests and approval procedures and the Chassieu site has developed specific skills in this field, including becoming a CBTL under the CB-MED and CB-EMC/MED scheme.
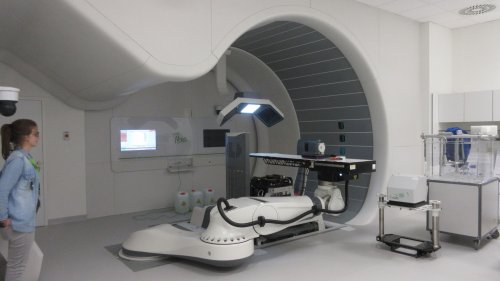
Mr. Henri-Louis Richard, head of the electrical safety laboratory at Emitech Chassieu, has been conducting medical equipment incorporation tests for the past four years in several countries around the world for his customer IBA. IBA is a leader in its field and has developed a revolutionary proton therapy system called Proteus 235®; a customized, intensity-modulated proton beam technology. Its protocols provide a solution for patients with a wide variety of complex cancer diseases. Proteus is more than a medical solution, it is a complete installation within a hospital building, with several treatment rooms and a particle accelerator shielded by thick concrete walls as radiological protection against the radiation induced by the operation of the accelerator.
An unprecedented form of test campaign, over a long period
Henri Louis Richard explains: “just one test campaign like this lasts about a year. The Proteus 235® system includes more than thirty electrical cabinets, a water-cooling room, numerous human-machine interfaces (HMIs), plus all the platform equipment itself.
That’s where the various systems for locating, visualizing and treating the tumour (X-rays, scanner, robot, radiology equipment) are interconnected. And we test all of these machine individually. In phase one, Emitech carried out initial tests directly in IBA’s site in Belgium, while the modules were under development, and then in its laboratories, it tests every single electrical component in each cabinet. Later, the complete installation is then inspected and tested in-situ for the final incorporation of the system. This takes into account the complete hardware integration and its installation (the network connection for example). The services we provide have to comply with national deviations from international standards (a kind of upgrading to local standards). Finally, we establish the corresponding normative documents for each country with the respective deviations. These tests are generally the same as in the laboratory but with different levels.”
For the past 2 years, IBA has been employing Emitech to perform test campaigns like these for sterilization equipment which function by means of similar electron acceleration processes at several MeV, known as Rhodotron®, Dynamitron® and Cyclotron. Mr. Richard adds, “These campaigns proceed in the same way. We carry out incorporation tests but the difference is that this type of equipment is classed as a machine. We therefore had to add to our tests the protocols related to the Machine Directive in addition to those for the Low Voltage Directive (electrical equipment safety requirements). The sterilization equipment must be checked in-situ because each configuration is unique in order to comply with the final customer’s specifications. These incorporation tests have been successfully carried out in about ten countries, including China, South Korea, the United States, the Dominican Republic, Vietnam and Italy.
To ensure proper management of the technical file, which is essential to any certification process, Emitech systematically proposes a pre-analysis of the product from the design phase onwards with respect to the requirements set by the applicable regulations (European directives, harmonised standards, national deviations, etc.) and the carrying out of pre-qualification tests to validate the solutions and technologies used before proceeding to the final tests.
“These international incorporation test campaigns conducted for our client IBA have been a real catalyst to structure our teams and processes,” adds Mr. Richard. The laboratory now carries out incorporation test campaigns for other customers who also have the specific requirement of deployment to different places around the world. In doing so, it strives to stay true to its slogan “We transform your tests into success” because success in these measurement campaigns is decisive for the marketing and commissioning of the systems.